Background: As one of the most prevalent pathogens in the environment, influenza affects millions of patients annually and contributed to tens of thousands of deaths in the United States during 2018-2019 alone. Immune deficiency is one of the most significant risk factors for adverse outcomes in adults with influenza, and similarly infectious complications are one of the most common causes of death in hematopoietic stem cell transplant (HSCT) recipients. However, data on outcomes or risk factors for progression in influenza infections specifically in HSCT recipients are limited.
Methods: We reviewed patient demographics, oncology and transplant history from HSCT recipients with polymerase chain reaction (PCR)-confirmed influenza over 5 influenza seasons (from 2013-2014 through 2018-2019). Influenza subtype (A H1, H3, and B), laboratory studies at diagnosis, treatments, vaccination status, and outcomes for each influenza infection were recorded and analyzed.
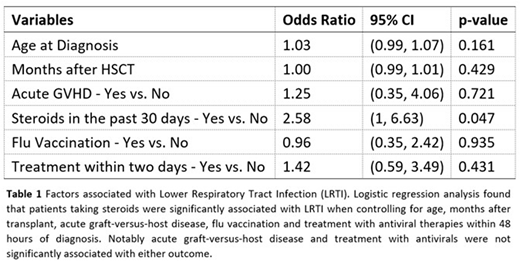
Results: A total of 143 infection events were collected and analyzed for symptoms and outcomes. Influenza infections of each subtype occurred in each season, although a different strain predominated each season. The most common symptoms at presentation were cough (65.3%) and fever (55.2%). Symptoms at presentation and outcomes were not significantly different amongst Influenza type A H1, A H3 and type B infections (p>0.05). In total, 22 (15.4%) infections presented as lower respiratory tract infections (LRTIs) and 7 additional infections progressed to LRTIs (all LRTI: 29; 20.1%). 63 (44.1%) infected patients required hospital admission, 15 (10.5%) required transfer to the intensive care unit (ICU) and 8 (5.6%) were intubated. 6 patients (4.2%) died by 30 days after the initial positive test, and 23 (16.1%) died by 180 days. LRTI was significantly associated with hospital admission (p<0.001), transfer to the ICU (p<0.001), intubation (p=0.005), and 30-day mortality (p=0.016); it was not significantly associated with 180-day mortality (0.357). The chronic use of steroids within the past 30 days prior to the initial positive test was significantly associated with risk of presenting with a LRTI (p=0.009), all LRTI (p=0.017), admission (p=0.010), and both 30-day (p=0.027) and 180-day mortality (p=0.016). It was not significantly associated with transfer to the ICU (p>0.05) or intubation (p>0.05). No other factors were significantly associated with LRTI, including vaccination status or treatment with antivirals (p>0.05). Logistic regression analysis of selected factors found that patients receiving steroids were significantly associated with LRTI (OR 2.58: 95% CI 1.00-6.63; p=0.047) (Table 1). There was, however, no statistically significant association detected between active graft-versus-host disease and LRTI (OR 1.25: 95% CI 0.35-4.06; p=0.721). We also found that treatment with oseltamivir within 2 days of symptom onset was not significantly associated with LRTI (OR 1.42: 95% CI 0.59-3.49; p=0.431). Persistent shedding (positive tests longer than 21 days from initial positive test) was also not associated with adverse outcomes (p>0.05).
Conclusion: This study suggests that the use of chronic steroids in treatment for graft-versus-host disease in HSCT patients may increase the risk for adverse outcomes in influenza infections.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal